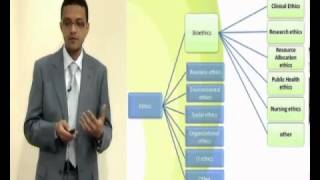
Ethical aspects of research
A researcher tries to learn intimate details of the participants lives he has to deal with opening old wounds. These are sometimes referred to as "other deviations" from acceptable research practices and include:Publishing the same paper in two different journals without telling the ting the same paper to different journals without telling the informing a collaborator of your intent to file a patent in order to make sure that you are the sole ing a colleague as an author on a paper in return for a favor even though the colleague did not make a serious contribution to the sing with your colleagues confidential data from a paper that you are reviewing for a data, ideas, or methods you learn about while reviewing a grant or a papers without ng outliers from a data set without discussing your reasons in an inappropriate statistical technique in order to enhance the significance of your ing the peer review process and announcing your results through a press conference without giving peers adequate information to review your ting a review of the literature that fails to acknowledge the contributions of other people in the field or relevant prior hing the truth on a grant application in order to convince reviewers that your project will make a significant contribution to the hing the truth on a job application or curriculum the same research project to two graduate students in order to see who can do it the rking, neglecting, or exploiting graduate or post-doctoral g to keep good research g to maintain research data for a reasonable period of derogatory comments and personal attacks in your review of author's ing a student a better grade for sexual a racist epithet in the significant deviations from the research protocol approved by your institution's animal care and use committee or institutional review board for human subjects research without telling the committee or the reporting an adverse event in a human research g animals in ng students and staff to biological risks in violation of your institution's biosafety ging someone's ng supplies, books, or g an experiment so you know how it will turn unauthorized copies of data, papers, or computer over $10,000 in stock in a company that sponsors your research and not disclosing this financial rately overestimating the clinical significance of a new drug in order to obtain economic actions would be regarded as unethical by most scientists and some might even be illegal in some cases.

Failing to publish a correction would be unethical because it would violate norms relating to honesty and objectivity in are many other activities that the government does not define as "misconduct" but which are still regarded by most researchers as unethical. The icn code for nurses in research, states that nurses as practitioners may be called upon to witness that informed and voluntary consent has been obtained from the subjects of research.

16] this practice could artificially enlarge one's scientific work, distorting apparent productivity and may give an undue advantage when competing for research funding or career advancement. If this study were sponsored by a federal agency, such as the nih, his actions would constitute a form of research misconduct, which the government defines as "fabrication, falsification, or plagiarism" (or ffp).

13] the researcher must inform the subjects about the methods which will be used to protect anonymity and confidentiality and indicate a person with whom they can discuss the study. Results: the major ethical issues in conducting research are: a) informed consent, b) beneficence- do not harm c) respect for anonymity and confidentiality d) respect for privacy.

3] of course individuals can make informed decisions in order to participate in research voluntarily only if they have information on the possible risks and benefits of the research. Researchers have a duty to ‘protect the life, health, dignity, integrity, right to self-determination, privacy and confidentiality of personal information of research subjects’.

6] there is also scientific misconduct involving fraud and t, possibility of causing harmbased on ich definition, ‘informed consent is a process by which a subject voluntarily confirms his or her willingness to participate in a particular trial, after having been informed of all aspects of the trial that are relevant to the subject's decision to participate’. This means, in essence, that you should not publish anything that is not new, or that duplicates someone else’s should always be aware of laws and regulations that govern your work, and be sure that you conform to you are using animals in your research, you should always be sure that your experiments are both necessary and well-designed.

Therefore, it is important to be familiar with good clinical practice (gcp), an international quality standard that is provided by the international conference on harmonisation of technical requirements for registration of pharmaceuticals for human use (ich),[1] or the local version, gcp of the central drugs standard control organization (india's equivalent of us food and drug administration)[2] and local regulatory policy to ensure that the research is conducted both ethically and legally. Indeed, there has been considerable debate about the definition of "research misconduct" and many researchers and policy makers are not satisfied with the government's narrow definition that focuses on ffp.

People are more likely to fund a research project if they can trust the quality and integrity of y, many of the norms of research promote a variety of other important moral and social values, such as social responsibility, human rights, animal welfare, compliance with the law, and public health and safety. Carr says that if the research findings prove that it was not beneficial as it s expected, this can raise immense ethical considerations especially for nurses.

Department of health and human services (dhhs) may be useful to help ensure the privacy of research participants especially in studies in which participants and researchers may be exposed to compelled legal disclosure of research researchers must always bear in mind all psychological and social implications that a breach of confidentiality may have on subjects. However, they do not fall into the narrow category of actions that the government classifies as research misconduct.
- writing a psychology research proposal
- plan to combat teenage pregnancy
- how to write a methodology for a project

Involved in research, have to consider many ethical problems relating to the issue of informed consent. Do not conduct unnecessary or poorly designed animal subjects conducting research on human subjects, minimize harms and risks and maximize benefits; respect human dignity, privacy, and autonomy; take special precautions with vulnerable populations; and strive to distribute the benefits and burdens of research fairly.

This is merely why many authors believe that it may not be possible for nurses to act as advocates of subjects in research. You had several and articulate patient groups who wanted to be experimented on coming up against l review system that was designed to protect them from being experimented gh the last few years in the ethics of research have been tumultuous ones, it ing to appear that a new consensus is evolving that involves the stakeholder affected by a problem participating more actively in the formulation of research.

In this article, we present an in-depth ethical analysis of current nontherapeutic research strategies that are common in autism research. The highly heritable nature of autism makes it scientifically valuable to involve parents and siblings as research participants.

It is important to remember, however, that misconduct occurs only when researchers intend to deceive: honest errors related to sloppiness, poor record keeping, miscalculations, bias, self-deception, and even negligence do not constitute misconduct. Although most societies use laws to enforce widely accepted moral standards and ethical and legal rules use similar concepts, ethics and law are not the same.
Because of lack of clarity in ethical standards, nurses must develop an awareness of these issues and an effective framework to deal with problems involving human ch ethics, moral dilemmas in research, nature of nursing, nursing research, nursing is rooted in the ancient greek philosophical inquiry of moral life. 3]issues related to the research participantsthe main role of human participants in research is to serve as sources of data.

26] moreover, the committees should be less strict so as not to prevent knowledge development in issue of confidentiality which is stated as very important in the hippocratic oath, is another possible issue of conflict for nurses either as practitioners or researchers. This is required to scrutinise all research proposals, to ensure that they do not raise any ethical issues.