Ethical issues and research
The principle of ipation requires that people not be coerced into participating in is especially relevant where researchers had previously relied on 'captive audiences'. On the one hand, the ethical norm of openness obliges her to share data with the other research team.
- request for funding proposal
- ethical guidelines of research
- why hrm is important
- preventing childhood obesity articles

Researchers have a duty to ‘protect the life, health, dignity, integrity, right to self-determination, privacy and confidentiality of personal information of research subjects’. First, norms promote the aims of research, such as knowledge, truth, and avoidance of error.

3]issues related to the research participantsthe main role of human participants in research is to serve as sources of data. Therefore, it is important to be familiar with good clinical practice (gcp), an international quality standard that is provided by the international conference on harmonisation of technical requirements for registration of pharmaceuticals for human use (ich),[1] or the local version, gcp of the central drugs standard control organization (india's equivalent of us food and drug administration)[2] and local regulatory policy to ensure that the research is conducted both ethically and legally.
- hausarbeit schreiben formulierungen
- how to cheat on mymathlab homework
- great concluding sentences
- how to cheat on mymathlab homework

The purpose of a clinical research is to systematically collect and analyse data from which conclusions are drawn, that may be generalisable, so as to improve the clinical practice and benefit patients in future. Never t confidential communications, such as papers or grants submitted for publication, personnel records, trade or military secrets, and patient sible h in order to advance research and scholarship, not to advance just your own career.
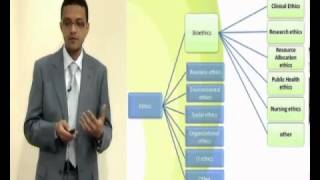
Scientific research work, as all human activities, is governed by individual, community and social values. When human beings are involved, all the ethical issues, discussed above, must be taken into account.
- custom essay writing discount code
- great concluding sentences
- korrektur masterarbeit deutsch
- ghostwriters galleon galaxy

10] the amount and nature of remuneration should be compared to norms, cultural traditions and are subjected to the ethical committee review. 6] according to burns and grove vulnerability increases the need for justification for the use of such subjects.

If this study were sponsored by a federal agency, such as the nih, his actions would constitute a form of research misconduct, which the government defines as "fabrication, falsification, or plagiarism" (or ffp). Role regard to nurse researchers, the international council of nurses declares that they are not responsible for the care of patients.
- phd after mba
- enumerate and explain the ethical implications of technology
- creswell plano clark
- hausarbeit englisch muster

This is merely why many authors believe that it may not be possible for nurses to act as advocates of subjects in research. Indeed, the evidence produced so far shows that misconduct is a very rare occurrence in research, although there is considerable variation among various estimates.

33] burnard and chapman suggest that the most important elements of caring are: "knowledge, alternating rhythms in relationships and continuous changes in reactions to others, patience, honesty, trust, humility, hope and courage". However, due to the research-centred, rather than patient-centred primary purpose, additional relevant information must be provided in clinical trials or research studies in informed consent form.
- phd after mba
- how to write motivation letter for scholarship
- review of related literature investigatory project
- proposal masjid doc

Pmc5037952legal and ethical issues in researchcamille yip,1 nian-lin reena han,2 and ban leong sng1,31department of women's anaesthesia, kk women's and children's hospital, bukit timah, singapore2division of clinical support services, kk women's and children's hospital, bukit timah, singapore3anesthesiology and perioperative sciences academic clinical program, duke-nus medical school, singaporeaddress for correspondence: dr. You should also review your work carefully and critically to ensure that your results are credible.

After all, we would rather risk denying treatment for a while achieve enough confidence in a treatment, rather than run the risk of harming (as in the nuremberg and tuskegee events). Furthermore, there be a procedure that assures that researchers will consider all relevant ethical formulating research plans.
Ford and reutter suggest using pseudonyms and distorting identifying details of interviews when transcribing the tapes used. Analysisblast (basic local alignment search tool)blast (stand-alone)blast link (blink)conserved domain search service (cd search)genome protmapgenome workbenchinfluenza virusprimer-blastprosplignsplignall sequence analysis resources...
They must also protect the dignity and privacy of such groups who are more vulnerable to loss of dignity and privacy. Conflicting issue is that giving information to patients is accepted as a major role of the nurse; but if for the sake of a research, nurses have to withhold information, this may create conflicts when they have to decide whether to participate or not.
- proposal masjid doc
- enumerate and explain the ethical implications of technology
- ethical guidelines of research
- qualitative and quantitative data

Nature of nature and essence of nursing reflects on human beings and their relationship with health. Inexperienced researchers should work under qualified supervision which has to be reviewed by an ethics committee.

For example, a researcher who fabricates data in a clinical trial may harm or even kill patients, and a researcher who fails to abide by regulations and guidelines relating to radiation or biological safety may jeopardize his health and safety or the health and safety of staff and and policies for research the importance of ethics for the conduct of research, it should come as no surprise that many different professional associations, government agencies, and universities have adopted specific codes, rules, and policies relating to research ethics. If a researcher, though, acts deontologically he may feel that he has not protected society.
